Answer:
D. 5
Step-by-step explanation:
![\mathbf{pH=-log_(10)[H^(+)]}](https://img.qammunity.org/2020/formulas/physics/middle-school/9klsr19l01nq4gxw5cni7vsrtelqexipr1.png)
The above formula is used to find pH of any solution. For that you require the concentration of
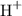 or hydronium ion (
or hydronium ion (
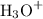 ) in the solution. Concentration should be in molarity (M) to get the pH.
) in the solution. Concentration should be in molarity (M) to get the pH.
The concentration of
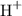 ion is given as 0.00001 M or
ion is given as 0.00001 M or
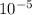 M.
M.
Substituting the value of
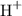 ion in the formula:
ion in the formula:
![\textrm{pH}=- \textrm{log}_(10)[10^(-5)]](https://img.qammunity.org/2020/formulas/physics/middle-school/sf0swh6tzeih0fewv1u6g4sz8gjf7mhlx5.png)
![\textrm{pH}=-(-5)\textrm{log}_(10)[10]](https://img.qammunity.org/2020/formulas/physics/middle-school/lwpoiauann3wmxq4e1h5w0k9xa21ee09zl.png)
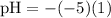
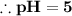
(NOTE : Some logarithmic properties
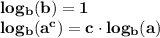
here 'b' is called the base of log and '
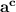 ' is called dthe argument of log. In the solution the base is '10' and the argument is
' is called dthe argument of log. In the solution the base is '10' and the argument is
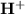 )
)