Answer:
Excess Reagent = oxygen
Step-by-step explanation:
Limiting reagent: The substance that is totally consumed when the reaction is completed.
Excess reagent: The substance left after the limiting reagent is consumed completely
The balanced chemical equation for formation of water is as follow:
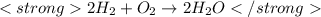
This means when 2 moles of hydrogen reacts with 1 mole of oxygen, 2 moles of water is produced.
Hence the ratio in which hydrogen and oxygen gas reacts is 2:1
Now if 2 mole hydrogen require 1 mole of oxygen ,then 4 mole hydrogen need 2 mole of oxygen.
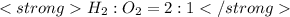
or
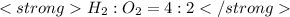
Here 5 mole of oxygen is reacting but only 2 mole is required .
Oxygen is in excess.