Answer:
D. -120.9 kJ
Step-by-step explanation:
According to Hess's law ,the total enthalpy change for a reaction is the sum of all changes regardless of the stages or the steps of the reaction.
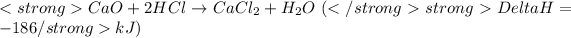 ....(1)
....(1)
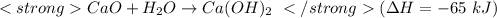
(this reaction should be reversed in order to reach the required reaction )
On reversing the reaction the sign of
 get reversed.
get reversed.
(In this case change sign from '-' to'+'. Hence
 = + 65 kJ)
= + 65 kJ)
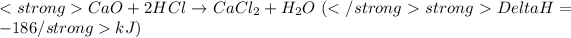 ....(1)
....(1)
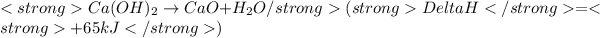 ......(2)
......(2)
Adding equation (1) and (2)
![<strong>Ca(OH)_(2) + 2HCl \rightarrow CaCl_(2) + 2H_(2)O</strong>[tex]</p><p>[tex]<strong>Delta H = - 186 + 65 = - 121\kJ</strong>](https://img.qammunity.org/2020/formulas/chemistry/middle-school/erkadr9avtpmlh3xnztx75vanug9kfo0q8.png)
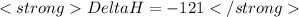 (It is nearly equal to -120.9 kJ)
(It is nearly equal to -120.9 kJ)