Step-by-step explanation:
Expression for
 of the given reaction is as follows.
of the given reaction is as follows.
![K_(sp) = [Cu^(2+)]^(2)[Fe(CN)_(6)]](https://img.qammunity.org/2020/formulas/chemistry/college/i54604tgljgan3rh2wazbk0e6qnc9q2m13.png)
Let us assume that the concentration of given species is "s". As the value of
 is given as
is given as
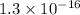 .
.
![K_(sp) = [Cu^(2+)]^(2)[Fe(CN)_(6)]](https://img.qammunity.org/2020/formulas/chemistry/college/i54604tgljgan3rh2wazbk0e6qnc9q2m13.png)
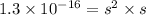
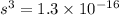
s =
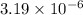
Therefore, concentration of
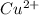 will be calculated as follows.
will be calculated as follows.
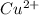 = 2s
= 2s
=
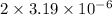
=
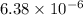 M
M
Now, we will calculate the value of
 as follows.
as follows.
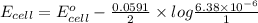
=
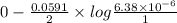
= 0.1535 V
Thus, we can conclude that the potential of given cell is 0.1535 V.