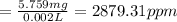Answer:
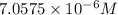 is the molar concentration of the solution.
is the molar concentration of the solution.
5.759 mg of crystal violet was dissolved to prepare the 2.00 mL sample that was measured in the cuvette.
Concentration of the solution in ppm is 2,879.31.
Step-by-step explanation:
Using Beer-Lambert's law :
Formula used :

where,
A = absorbance of solution
C = concentration of solution
 = The molar absorptivity coefficient
= The molar absorptivity coefficient
We have :
A = 0.614 , l = 1.0 cm , C= ?
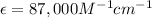
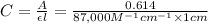
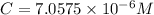
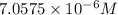 is the molar concentration of the solution.
is the molar concentration of the solution.
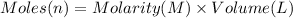
Moles of crystal violet = n
Volume of crystal violet solution = 2.00 mL = 0.002 L
Molarity of the crystal violet =
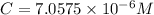
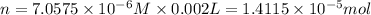
Mass of
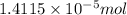 of crystal violet:
of crystal violet:
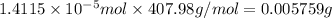
0.005759 g = 5.759 mg (1 g = 1000 mg)
5.759 mg of crystal violet was dissolved to prepare the 2.00 mL sample that was measured in the cuvette.
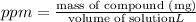
Mass of crystal violet = 5.759 mg
Volume of solution= 2 mL = 0.002 L
Concentration of the solution in ppm: