Answer:
final pressure would be p2 = 10.833 atm
Step-by-step explanation:
If the gas has constant temperature then pressure×volume = constant.
let the initial pressure and volume be p1, v1
and final pressure and volume be p2, v2.
therefore
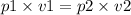
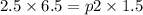
therefore final pressure would be p2 = 10.833 atm