Answer:
35 g of Oxygen will give 59.7 g of SeO3
Step-by-step explanation:
Atomic number of SeO3 = 34 + 3×16 = 82 g
In this reaction 3 moles of oxygen gives 2 moles of SeO3
therefore one mole of oxygen will give
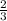 moles of SeO3
moles of SeO3
that is 32 g of oxygen will give
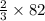 g of SeO3
g of SeO3
therefore 35 g of oxygen will give
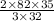 g of SeO3
g of SeO3
=59.7 g of SeO3