Answer:
The melting points of the second series of transition elements are electrons are entering to the type of orbital.
Step-by-step explanation:
Transition elements have "d" band that can overlap with the "s' band to give a composite band. The composite band have six molecular orbitals per metal atom.
Half of the molecular orbitals are the anti bonding.So, maximum bonding occurs when the molecular orbitals contains six electrons i.e. the metal has six valence electrons per each metal atom.
When the maximum bonding occurs then it shows maximum melting point.
So, molybdenum shows melting point at
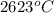 and after that the electrons goes into the antibonding and reaches minimum for cadmium when the anti boning orbital is completely filled. So, the melting point of the cadmium is tex]321^{o}C[/tex].
and after that the electrons goes into the antibonding and reaches minimum for cadmium when the anti boning orbital is completely filled. So, the melting point of the cadmium is tex]321^{o}C[/tex].