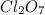Answer:
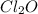 ,
,
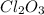 ,
,
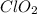 ,
,
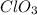 ,
,
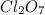
Step-by-step explanation:
Empirical formula of the compound is the simplest ratio of elements present in the compound.
Empirical formula of compounds of chlorine with oxygen is as follows:
Compounds in which oxidation state of Cl is +1
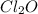
Compounds in which oxidation state of Cl is +3
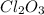
Compounds in which oxidation state of Cl is +4
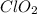
Compounds in which oxidation state of Cl is +6
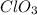
Compounds in which oxidation state of Cl is +7