Answer:
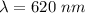
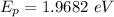
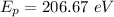 is the photon energy of an x-ray
is the photon energy of an x-ray
Step-by-step explanation:
Given:
Energy of photon,
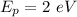
Then according to given:
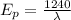
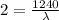
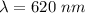 is the wavelength
is the wavelength
Again,
Energy of photon,
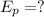
wavelength of the light,
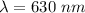
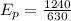
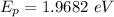
Now, photon energy for an x-ray:
wavelength,
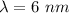
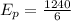
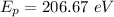 is the photon energy of an x-ray
is the photon energy of an x-ray