Answer:
option D denotes electron in lowest energy level.
Step-by-step explanation:
the energy of an electron in hydrogen orbit is given by
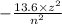 where z is the atomic number and n is the energy level or orbit number . therefore as the n increases energy also increases.
where z is the atomic number and n is the energy level or orbit number . therefore as the n increases energy also increases.