Answer:
The bonds which are broken are:-
Bonds of
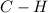 in the
in the

Bonds of
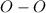 in the
in the

Bonds which are formed are:-
Bonds of
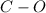 in the
in the

Bonds of
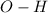 in the
in the

Step-by-step explanation:
The combustion reaction of methane is shown below as;-
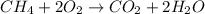
Covalent bond is the bond which is formed with the sharing of the electrons between the two atoms which are taking part in the bond. It is generally formed between the atoms with similar electronegativity values. All the bonds in the above equation are covalent bonds.
The bonds which are broken are:-
Bonds of
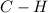 in the
in the

Bonds of
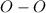 in the
in the

Bonds which are formed are:-
Bonds of
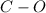 in the
in the

Bonds of
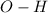 in the
in the
