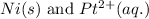Answer: The combination of element ad an ion that will react is
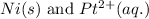
Step-by-step explanation:
Oxidation reaction is defined as the reaction in which an atom looses its electrons. The oxidation number of the atom gets increased during this reaction.
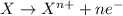
Reduction reaction is defined as the reaction in which an atom gains electrons. The oxidation number of the atom gets reduced during this reaction.
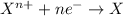
The substance having highest positive
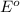 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.
For a reaction to be spontaneous, the standard electrode potential must be positive.
To calculate the
 of the reaction, we use the equation:
of the reaction, we use the equation:
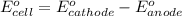 ......(1)
......(1)
For the given options:
- Option 1:
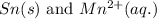
Here, tin must undergo oxidation reaction and manganese undergo reduction reaction.
Oxidation half reaction:
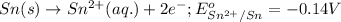
Reduction half reaction:
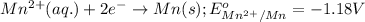
Putting values in equation 1, we get:
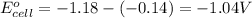
As, the standard potential is coming out to be negative, the given reaction will not take place.
- Option 2:
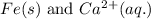
Here, iron must undergo oxidation reaction and calcium undergo reduction reaction.
Oxidation half reaction:
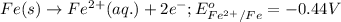
Reduction half reaction:
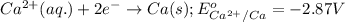
Putting values in equation 1, we get:

As, the standard potential is coming out to be negative, the given reaction will not take place.
- Option 3:
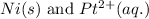
Here, nickel must undergo oxidation reaction and platinum undergo reduction reaction.
Oxidation half reaction:
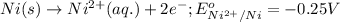
Reduction half reaction:
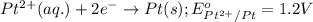
Putting values in equation 1, we get:
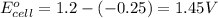
As, the standard potential is coming out to be positive, the given reaction will take place.
- Option 4:
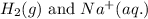
Here, hydrogen must undergo oxidation reaction and sodium undergo reduction reaction.
Oxidation half reaction:
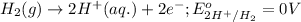
Reduction half reaction:
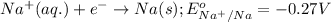
Putting values in equation 1, we get:
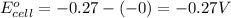
As, the standard potential is coming out to be negative, the given reaction will not take place.
Hence, the combination of element ad an ion that will react is