Answer:
amount of water produced = 31.76 g of water
Step-by-step explanation:
20 g of ammonia has
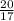 moles of ammonia
moles of ammonia
and 50 g of Oxygen has
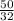 moles of oxygen .
moles of oxygen .
to find the limiting agent we have to find the mole ratio
mole ratio of ammonia =
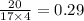
mole ratio of oxygen =
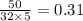
therefore the limiting reagent is ammonia.
4 moles ammonia gives 6 moles of water
therefore 1 mole of ammonia gives
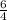 moles of water
moles of water
therefore
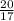 moles of ammonia gives
moles of ammonia gives
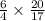 moles of water.
moles of water.
amount of water produced = 1.76 moles of water = 31.76 g of water