Answer:
Energy released:
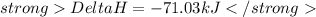
Step-by-step explanation:
In Haber's process Ammonia formation takes place according to equation:
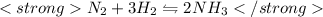
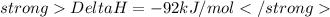
According to this equation ,when 2 mole of ammonia is formed from 3 mole of nitrogen and 1 mole of oxygen then 92 kJ of heat is released.
1 mole of
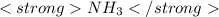 contains 17.03 g
contains 17.03 g
mass of N=14.0067 g/mol
mass of H=1.007 g/mol
mass of
 =14.0067+3.021
=14.0067+3.021
mass of
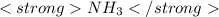 =17.03 g/mol
=17.03 g/mol
So, 2 mole of
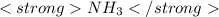 contains
contains
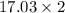 =34.06g
=34.06g
Since 34.06g (or 2mol) = -92 kJ
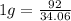
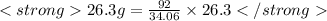
this gives
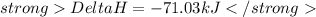
Hence amount of energy released when 26.3g of ammonia is formed is -71.03 kJ