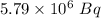Answer:
Step-by-step explanation:
Calculation of the moles of sodium perchlorate as:-
Mass = 54.3 mg
Also, 1 mg = 0.001 g
So, Mass =
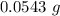
Molar mass of sodium perchlorate = 122.44 g/mol
The formula for the calculation of moles is shown below:

Thus,
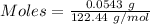
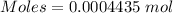
Also, given that it contains 29.6 % of the radioactive Chlorine
So, Moles of radioactive chlorine in the sample =
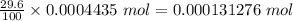
1 mole of Chlorine contains
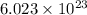 atoms of chlorine
atoms of chlorine
So,
0.000131276 mole of Chlorine contains
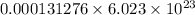 atoms of chlorine
atoms of chlorine
Atoms of radioactive chlorine in the sample =
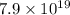
Given that:
Half life =
 year
year
1 year =
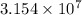 s
s
Half life =
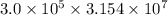 s = 9462000000000 s
s = 9462000000000 s
The expression for half-life is:-
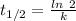
Where, k is rate constant
So,
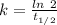
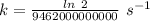
The rate constant, k =
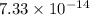 s⁻¹
s⁻¹
Disintegration is:-
Disintegrations per second = Rate constant*Number of atoms =
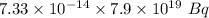 =
=