Answer:
1·199 J
Step-by-step explanation:
Given
Mass of water = 0·814 g = 0·814 ×
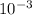 kg
kg
Increase in temperature = 0·351 °C
Let the amount of heat added be Q J
Formula for heat added is
Q = m × s × ΔT
where Q is the amount of heat transferred
m is the mass
s is the heat capacity
ΔT is the change in temperature
Heat capacity of water = 4200 J/kg °C
Applying the formula for heat added
Q = 0·814 ×
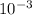 × 4200 × 0·351 = 1·199 J
× 4200 × 0·351 = 1·199 J
∴ Amount of heat added = 1·199 J