Answer:
Molly has to add 22.86 milliliters of 5% sulfuric acid.
Explanation:
Molly has a mixture of 20 ml of 20% sulfuric acid solution.
She mixes this with 5% sulfuric acid solution and in the end she wants a 12% sulfuric acid solution.
We have to find how many milliliters of 5% sulfuric acid we have to add to make it 12% solution.
Let the volume we add be v milliliters.
We are going to equate sulfuric acid.
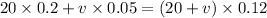
v =
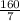 milliliters
milliliters
v = 22.86 milliliters