Answer:
22.86 ml.
Explanation:
Let x ml of 5% sulfuric solution must Molly add to 20ml of 20% solution to get a 12% solution.
Now, x ml of 5% sulfuric acid solution has pure acid of
 ml.
ml.
Again, 20 ml of 20% sulfuric acid solution has pure acid of
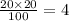 ml.
ml.
Therefore, we can write the equation as
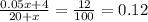
⇒ 0.05x + 4 = 2.4 + 0.12x
⇒ 0.07x = 1.6
⇒ x = 22.86 ml. (Answer) (Approximate value)