Answer:
option C
Step-by-step explanation:
given,
Period of oxygen molecule = 6 s
number of molecule = 6 x 10²³
Area of wall = 2 cm²
speed of molecule = 400 m/s
mass of O₂ molecule = 5.344 × 10⁻²⁶ Kg
we know,
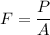
Δp= F Δt
Δp change in momentum
Δp = N.m.Δv
where N is the number of oxygen molecules strike a wall
Δv = v - (-v)= 2 v
Pressure,
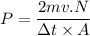
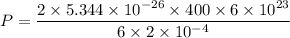
P = 21376 Pa
P = 21.4 kpa
hence the correct answer is option C