Answer:
density of oxygen = 1.307 g/l
Step-by-step explanation:
given pressure = 1 atm temperature = 298 k
PM=DRT
where p is pressure M is molecular mass D is density R is gas constant and T is temperature
substituting the values in the equation
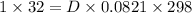
D = 1.307 g/l.