Answer:
772 nanometers.
Step-by-step explanation:
The maximum wavelength of light corresponds to minimum energy required to break one mole of flourine-flourine single bond which is 155 kJ.
Energy required to break one F-F bond is
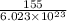 kJ.
kJ.
Energy of photon is given by:
E=\frac{nhc}{\lambda}[/tex]
where
n = number of photons h=
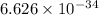 Js and
Js and
 =wavelength of photon and c=
=wavelength of photon and c=
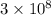
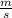
In this question E=
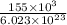
and n=1
Substituting appropriate values in the above equation, we get:
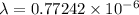 metres
metres
=772.42 nanometers
=772 nanometers as value given in the question has 3 significant digits.