Answer:
1/12 of the mass of a ¹²C atom is exactly 1u. Therefore, the mass of the hydrogen atom is greater than 1/12 of the mass of a ¹²C atom.
Step-by-step explanation:
The unified atomic mass unit is defined as 1/12 of the mass of a ¹²C atom:
1u = 1/12 m ¹²C
Also, the unified atomic mass unit is equal to the mass (in grams) of one mole of a substance, so knowing that 1 mol is equal to 6.022x10²³ atoms, the unified atomic mass unit measured in grams is:
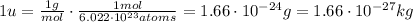
Starting from this definition, we can calculate the mass of ¹H atom respect to the mass of a ¹²C atom:
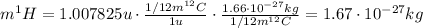
Therefore, the mass of a ¹H atom is greater than 1/12 the mass of a ¹²C atom.
I hope it helps you!