Answer:
The possible structures are ketone and aldehyde.
Step-by-step explanation:
Number of double bonds of the given compound is calculated using the below formula.
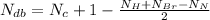
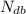 =Number of double bonds
=Number of double bonds
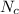 = Number of carbon atoms
= Number of carbon atoms
 = Number of hydrogen atoms
= Number of hydrogen atoms
 = Number of nitrogen atoms
= Number of nitrogen atoms
The number of double bonds in the given formula -
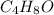
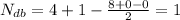
The number of double bonds in the compound is one.
Therefore, probable structures is as follows.
(In attachment)
The structures I and III are ruled out from the probable structures because the signal in 13C-NMR appears at greater than 160 ppm.
alkene compounds I and II shows signal less than 140 ppm.
Hence, the probable structures III and IV are given as follows.
The carbonyl of structure I appear at 202 and ketone group of IV appears at 208 in 13C, which are greater than 160.
Hence, the molecular formula of the compound
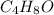 having possible structure in which the signal appears at greater than 160 ppm are shown aw follows.
having possible structure in which the signal appears at greater than 160 ppm are shown aw follows.