Answer:
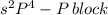
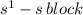
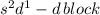
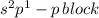
Step-by-step explanation:
The given valence electronic configurations is as follows.
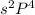
In this configuration, completely filled s-orbital and electrons are enters into the p-bolck therefore, it is p-block configuration.
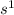
In this configuration, electrons are enters into the s-bolck therefore, it is s-block configuration.
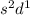
In this configuration, completely filled s-orbital and electrons are enters into the d-bolck therefore, it is d-block configuration.
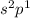
In this configuration, completely filled s-orbital and electrons are enters into the p-bolck therefore, it is p-block configuration.