Answer:
Step-by-step explanation:
Given
Temperature of Hot reservoir
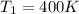
Temperature of Cold reservoir
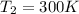
Heat reservoir
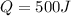
Work done
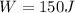
heat Exhaust
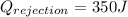
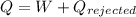
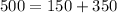
L.H.S=R.H.S
thus it follows first law
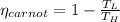
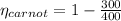
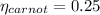
Cycle Efficiency
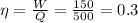
Carnot efficiency is less than cycle thus it violates second law