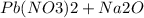Answer:
The balanced reaction is given by,
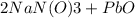 ⇒
⇒
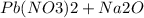
Step-by-step explanation:
The reaction is as given.
Lets count the number of each elements in the reaction.
In reactant side, number of sodium atoms are 1 , lead are 1, nitrogen are 1 and oxygen are 4.
in product side, number of sodium atoms are 2 , lead are 1 , nitrogen are 2 and oxygen are 7.
So we need to balance sodium and oxygen atoms in the reaction.
There is deficient of sodium and oxygen atoms on reactant side.
Thus, multiply (NaNO3) by 2.
Thus, sodium atoms become 2 , nitrogen 2 and oxygen 6. Total 7 oxygen atoms.
Thus, the balanced reaction is,
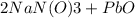 ⇒
⇒