Answer:
a) equal 1, b) less than 1
Step-by-step explanation:
a) the electric force is given by
Fe = q E
The charge of the electron and proton has the same value, that of the proton is positive and that of the electron is negative
Proton
Fp = qE
Electron
Fe = - q E
Fp / Fe = -1
If we do not take into account the sign the relationship is equal to one (1)
b) to calculate the force we use Newton's second law
F = ma
qE = m a
a = q E / m
The mass of the proton much greater than the mass of the electron
ap = q E /
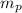
ae = - q E /
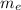
ap / ae =
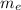 /
/
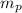 =
=
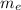 /1600
/1600
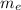 =1/1600
=1/1600
It is much smaller than 1