Answer:
The pressure of dry hydrogen gas is 734 mm Hg.And number of moles of hydrogen gas is 0.017mol.
Reacted mass of aluminium is 0.306 g.
Step-by-step explanation:
The total pressure is the sum of the individual pressure.
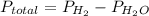
Total pressure = 755 mmHg
Pressure of water = 21mmHg
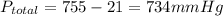
Therefore, The pressure of dry hydrogen gas is 734 mm Hg.
Number of moles of hydrogen gas can be calculate is as follows.
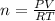
From the given,
Pressure "P" = 755 mmHg = 0.993 atm
Volume"V"= 415 mL = 0.415 L
Gas constant "R" = 0.0821 L.atm/mol.K
Temperature = 23+273= 296 K
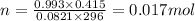
Therefore, number of moles of hydrogen gas is 0.017mol.
Mass of aluminium reacted:
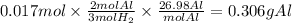
Therefore, Reacted mass of aluminium is 0.306 g.