Answer:
20%
Step-by-step explanation:
molecular formula of deuterium oxide, or "heavy water" is
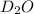
molecular weight of D2= 4.028204 g/mol
molecular weight of oxygen= 16 g/mol
therefore, molecular weight of water = 20.028204 g/mol
therefore, the percent hydrogen (by mass) in a "heavy water" molecule
=(molecular weight of D2/molecular weight of water)
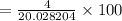
= 20%