Answer:
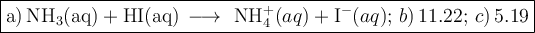
Step-by-step explanation:
a) Balanced equation
The balanced chemical equation for the titration is

b) pH at start
For simplicity, let's use B as the symbol for NH₃.
The equation for the equilibrium is
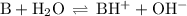
(i) Calculate [OH]⁻
We can use an ICE table to do the calculation.
B + H₂O ⇌ BH⁺ + OH⁻
I/mol·L⁻¹: 0.150 0 0
C/mol·L⁻¹: -x +x +x
E/mol·L⁻¹: 0.150 - x x x
![K_{\text{b}} = \frac{\text{[BH}^(+)]\text{[OH}^(-)]}{\text{[B]}} = 1.8 * 10^(-5)\\\\(x^(2))/(0.150 - x) = 1.8 * 10^(-5)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/2hdat9r30obwd1m7g607pnx1jy65p458bn.png)
Check for negligibility:
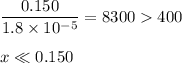
(ii) Solve for x
![(x^(2))/(0.150) = 1.8 * 10^(-5)\\\\x^(2) = 0.150 * 1.8 * 10^(-5)\\x^(2) = 2.7 * 10^(-6)\\x = \sqrt{2.7 * 10^(-6)}\\x = \text{[OH]}^(-) = 1.64 * 10^(-3) \text{ mol/L}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/ijudkt7qp4oqbp7j5gayu6zdb7s2k5j2bz.png)
(iii) Calculate the pH
![\text{pOH} = -\log \text{[OH}^(-)] = -\log(1.64 * 10^(-3)) = 2.78\\\\\text{pH} = 14.00 - \text{pOH} = 14.00 - 2.78 = \mathbf{11.22}\\\\\text{The pH of the solution at equilibrium is } \large \boxed{\mathbf{11.22}}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/42hpi8o0eilfmcgisgwje8x4ly7h8z3xr0.png)
(c) pH at equivalence point
(i) Calculate the moles of each species
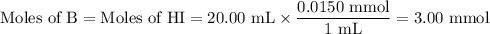
B + HI ⇌ BH⁺ + I⁻
I/mol: 3.00 3.00 0
C/mol: -3.00 -3.00 +3.00
E/mol/: 0 0 3.00
(ii) Calculate the concentration of BH⁺
At the equivalence point we have a solution containing 3.00 mmol of NH₄I
Volume = 20.00 mL + 20.00 mL = 40.00 mL
![\rm [BH^(+)] = \frac{\text{3.00 mmol}}{\text{40.00 mL}} = \text{0.0750 mol/L}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/hueeng8mq252ix2i2vurpjm8nganmmazwh.png)
(iii) Calculate the concentration of hydronium ion
We can use an ICE table to organize the calculations.
BH⁺+ H₂O ⇌ H₃O⁺ + B
I/mol·L⁻¹: 0.0750 0 0
C/mol·L⁻¹: -x +x +x
E/mol·L⁻¹: 0.0750 - x x x
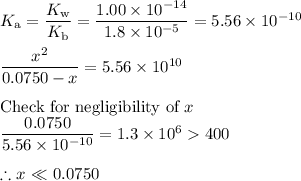
![(x^(2))/(0.0750) = 5.56 * 10^(-10)\\\\x^(2) = 0.0750 * 5.56 * 10^(-10)\\x^(2) = 4.17 * 10^(-11)\\x = \sqrt{4.17 * 10^(-11)}\\\rm [H_(3)O^(+)] =x = 6.46 * 10^(-6)\, mol \cdot L^(-1)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/hytcl7cm28d3fbmcqya6yob7vi02pdi3gt.png)
(iv) Calculate the pH
![\text{pH} = -\log{\rm[H_(3)O^(+)]} = -\log{6.46 * 10^(-6)} = \large \boxed{\mathbf{5.19}}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/m0s2id5qp3yp2votx3s2wrloqzdflh71fd.png)
The titration curve below shows the pH at the beginning and at the equivalence point of the titration.