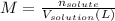Answer:
molality, mole fraction, solvent, solute, molarity.
Step-by-step explanation:
The expression of concentration that provides the moles of solute per kilograms of solvent is molality.
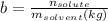
If you place 5 moles of sodium chloride and 4 moles of sucrose into 11 moles of water, the mole fraction of sodium chloride would be 0.25
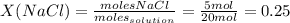
A solution is made up of 0.15 grams of sodium chloride in 1 liter of water. For this solution, the solvent is water.
The solvent is the component in major proportion and the one that defines the state of the solution.
A solution is made up of 0.15 grams of sodium chloride in 1 liter of water. For this solution, the solute is sodium chloride.
The solute is the component in minor proportion.
A way to express concentration that provides the moles of solute per liter of solution is molarity.