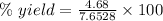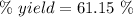Answer:
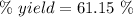
Step-by-step explanation:
1 mole of STP has a volume of 22.4 L
So,
Number of the moles of
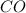 which are being fed per minute =
which are being fed per minute =
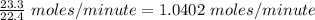
Also, Number of the moles of
 which are being fed per minute =
which are being fed per minute =
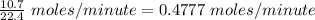
According to the reaction shown below:-
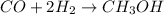
1 mole of
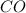 reacts with 2 moles of
reacts with 2 moles of

1.0402 mole of
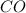 reacts with 2*1.0402 moles of
reacts with 2*1.0402 moles of

Moles of
 = 2.0804 moles
= 2.0804 moles
Thus,
 is the limiting reagent.
is the limiting reagent.
The formation of the product is governed by the limiting reagent. So,
2 moles of
 on reaction forms 1 mole of
on reaction forms 1 mole of
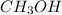
1 mole of
 on reaction forms 1/2 mole of
on reaction forms 1/2 mole of
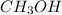
Also,
0.4777 mole of
 on reaction forms
on reaction forms
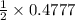 mole of
mole of
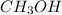
Moles of
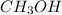 = 0.23885 moles
= 0.23885 moles
Also, Molar mass of
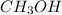 = 32.04 g/mol
= 32.04 g/mol
Mass = Moles*Molar mass = 0.23885*32.04 g = 7.6528 g
The expression for the calculation of the percentage yield for a chemical reaction is shown below as:-
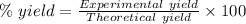
Given , Values from the question:-
Theoretical yield = 7.6528 g
Experimental yield = 4.68 g
Applying the values in the above expression as:-