Answer:
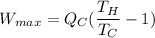
Step-by-step explanation:
given,
Temperature of heat engine operate between
Th (temperature in hot reservoir) and Tc(temperature in cold reservoir)
amount of heat released to = Qc
to find maximum amount of work = ?
now,
efficiency of heat engine
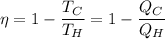
now,
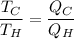
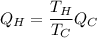
maximum work =
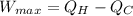
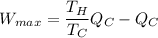
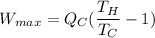
above expression gives the expression of maximum amount of work.