Answer:
The amount nitrogen gas in the given balloon = 23.07 g
Step-by-step explanation:
Given: Total volume of balloon: V = 18 L, Pressure = 1.4 atm, Temperature = 25°C = 25 + 273 = 298K (∵ 0°C = 273 K)
Volume of Nitrogen gas in balloon: V₁ = V × 80% = 18 L × (80 / 100) = 14.4 L
Molar mass of nitrogen gas (N₂): M = 28 g/mol
According to the ideal gas law:
PV= nRT
Here, Gas constant: R = 0.08206 L·atm/(mol·K)
n = total number of moles of gas
P = Pressure in atm
T = Temperature in K
V = Volume in L
Therefore, the number of moles of oxygen gas (n₁) is given by:
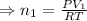
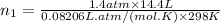
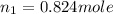
As number of moles:
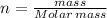
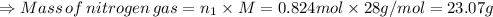
Therefore, the amount nitrogen gas in the given balloon = 23.07 g