Answer:
a)
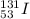
b)
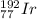
c)
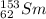
Step-by-step explanation:
The symbols of the isotopes are written like
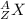
where,
X is the element
A is the mass number (protons + neutrons)
Z is the atomic number (protons)
a) Iodine-131
The atomic number of iodine is 53. The mass number of this isotope is 131. The symbol is
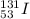 .
.
b) Iridium-192
The atomic number of iridium is 77. The mass number of this isotope is 192. The symbol is
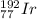 .
.
c) Samarium-153
The atomic number of samarium is 62. The mass number of this isotope is 153. The symbol is
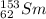 .
.