Answer:
8.18 M
Step-by-step explanation:
The osmotic pressure (π) is a colligative property that can be calculated using the following expression.
π = M . R . T
where,
M: molarity
R: ideal gas constant
T: absolute temperature
The absolute temperature is 25 + 273 = 298 K.
The osmotic pressure (in atm) is:
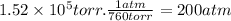
π = M . R . T
200 atm = M . (0.08206 atm.L/mol.K) . 298 K
M = 8.18 mol/L