Answer: The value of
 is 0.044
is 0.044
Step-by-step explanation:
We are given:
Initial moles of methane =
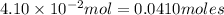
Initial moles of carbon tetrachloride =
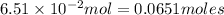
Volume of the container = 1.00 L
Concentration of a substance is calculated by:
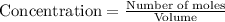
So, concentration of methane =
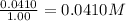
Concentration of carbon tetrachloride =
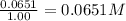
The given chemical equation follows:
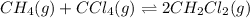
Initial: 0.0410 0.0651
At eqllm: 0.0410-x 0.0651-x 2x
We are given:
Equilibrium concentration of carbon tetrachloride =
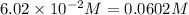
Evaluating the value of 'x', we get:
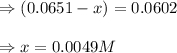
Now, equilibrium concentration of methane =
![0.0410-x=[0.0410-0.0049]=0.0361M](https://img.qammunity.org/2020/formulas/chemistry/college/jonluxhnntcz4ljum2mcq6mn2b8c6s3h5p.png)
Equilibrium concentration of
![CH_2Cl_2=2x=[2* 0.0049]=0.0098M](https://img.qammunity.org/2020/formulas/chemistry/college/m4dk1ecv1jkr28fdhx8f93yh3wil39i09j.png)
The expression of
 for the above reaction follows:
for the above reaction follows:
![K_(eq)=([CH_2Cl_2]^2)/([CH_4]* [CCl_4])](https://img.qammunity.org/2020/formulas/chemistry/college/xdo989ro9750ngs9c6h1hi2ckuczl13btr.png)
Putting values in above expression, we get:
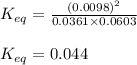
Hence, the value of
 is 0.044
is 0.044