Answer:
1,45 mL
Step-by-step explanation:
The Grignard reaction is a very important organometallic chemical reaction where the Grignard reagent ( alkyl, vinyl, or aryl-magnesium halides) acts as nucleophile in order to the formation of Carbon-Carbon bonds.
In the problem, the phenylmagnesium chloride is the grignard reagent. The volume of 2.0 M phenylmagnesium chloride solution you need to add 2.9 mmol is:
2,9 mmol ×
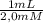 = 1,45 mL
= 1,45 mL
I hope it helps!