Answer:
The answer to your question is Molarity = 0.0708
Step-by-step explanation:
Data
NaOH 0.05 M Volume 1 = 3.87 ml Volume 2 = 25.11 ml
HCl 15 ml
Process
1.- Find the volume used of NaOH
25.11 - 3.87 = 21.24 ml = 0.02124 l
2.- Write the balanced equation of the reaction
NaOH + HCl ⇒ NaCl + H₂O
3.- Calculate the moles of NaOH in the solution
Molarity =
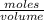
moles = Molarity x volume
moles = 0.05 x 0.02124
moles = 0.001062
4.- From the reaction we know that NaOH and HCl react in a proportion 1:1.
1 mol of NaOH ------------- 1 mol of HCl
0.001062 moles of NaOH ------------ x
x = (0.001062 x 1) / 1
x = 0.001062 moles of HCl
5.- Find the molarity of HCl
Molarity =
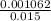
Molarity = 0.0708