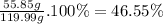Answer:
46.55%
Step-by-step explanation:
The molar ratio is 1 mole of iron to 2 moles of sulfur, which means that the empirical formula is FeS₂.
The mass of Fe in 1 mole of FeS₂ is 55.85 g.
The mass of S in 1 mole of FeS₂ is 2 × 32.07 g = 64.14 g.
The mass of 1 mole of FeS₂ is 55.85 g + 64.14 g = 119.99 g.
The mass percent of Fe in FeS₂ is: