Grams of Chromium(III) nitrate produced : 268.95 g
Further explanation
Given
0.85 moles of Lead(IV) nitrate
Required
grams of Chromium(III) nitrate
Solution
Reaction
Balanced equation :
2Cr₂ + 3Pb(NO₃)₄ ⇒ 4Cr(NO₃)₃ + 3Pb
From the equation, mol ratio of Pb(NO₃)₄ : Cr(NO₃)₃ = 3 : 4, so mol Cr(NO₃)₃
mol Cr(NO₃)₃ =
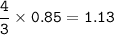
Mass of Chromium(III) nitrate (MW=238.0108 g/mol) :
mass = mol x MW
mass = 1.13 x 238.0108
mass = 268.95 g