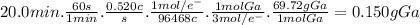Answer:
0.150 g
Step-by-step explanation:
First, we will write the reduction half-reaction to produce Ga(s)
Ga³⁺(aq) + 3 e⁻ → Ga(s)
We can establish the following relations:
- 1 min = 60 s
- 1 A = 1 c/s
- 1 mole of e⁻ has a charge of 96468 c (Faraday's constant)
- 1 mole of Gas(s) is deposited when 3 moles of e⁻ circulate.
- The molar mass of Ga is 69.72 g/mole.
The mass of Ga(s) deposited when a current of 0.520 A flows for 20.0 min is: