Answer:
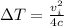
Step-by-step explanation:
Let the mass of bullet is m, initial velocity of bullet is vi and c be the specific heat of the bullet.
Kinetic energy, K = 1/2 mvi^2
According to the question, 50% of the kinetic energy is equal to the heat energy absorbed by the bullet.
50% of K = mass of bullet x specific heat x rise in temperature
1/4 mvi^2 = m x c x ΔT