Answer:
1976
Step-by-step explanation:
The first order decay of tritium can be represented through the following expression.
![ln(([H]_(t))/([H]_(0)) )=-k.t](https://img.qammunity.org/2020/formulas/chemistry/college/lt0w5w5zxhm88vdsro0vr9m908yym52t7g.png)
where,
[H]t is the concentration of tritium after a time t has elapsed
[H]₀ is the initial concentration of tritium
k is the rate constant
Given the half-life (t1/2) is 12.3 years, we can calculate the rate constant using the following expression.
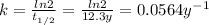
The concentration of tritium at certain time is 16% of the initial concentration, that is, [H]t = 0.16 [H]₀.
![ln((0.16[H]_(0))/([H]_(0)) )=-0.0564y^(-1).t\\t=32y](https://img.qammunity.org/2020/formulas/chemistry/college/xkfsiwic4njh2ei2oivp4mnk0omeie20l2.png)
If the watch was made in 1944, you could read the time until 1944 + 32 = 1976.