Answer:
Due to presence of unpaired electrons in outermost shell of oxygen, oxygen is a fairly reactive element while neon is not.
Step-by-step explanation:
Lets look at the electronic configuration of both the elements.
Oxygen, a chalcogen, has EC as =
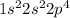
while, for Neon, a noble gas, EC is =
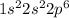
In neon, the outermost orbital p is completely filled while in oxygen it is partially filled, with 2 unpaired electrons.
Thus the completely filled orbitals give extra stability to neon and thus less reactivity. In other case, oxygen has 2 unpaired electrons, unstable and has exchange energy, making it more reactive.
Thus, oxygen is a fairly reactive element while neon is not..