To solve this problem it is necessary to apply the concepts concerning the conservation of both potential and thermodynamic energy of the body. That is to say that as the body has a loss of potential energy it is gained in the form of thermal energy on water. If the potential energy is defined as
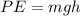
Where,
m= mass
g = Gravitational acceleration
h = Height
And thermal energy is obtained as
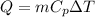
Where,
 = Change in Temperature
= Change in Temperature
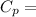 Specific Heat
Specific Heat
m = Mass
We can equate this equation and rearrange to find the change at the Temperature, then
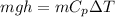
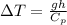
Our values are given as,
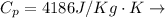 Specific Heat Water
Specific Heat Water
Using energy conservation
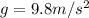
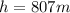
Replacing,
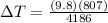
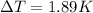
Therefore the temperature increase in a 1kg sample of water is 1.89K