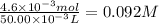Answer:
0.092 M
Step-by-step explanation:
Let's consider the following redox reaction.
8 H⁺ + MnO₄⁻ + 5 Fe²⁺ → 5 Fe³⁺ + Mn²⁺ + 4 H₂O
The moles of MnO₄⁻ is:

The molar ratio of MnO₄⁻ to Fe²⁺ is 1:5. Then, the moles of Fe²⁺ are:
5 × (9.1 × 10⁻⁴ mol) = 4.6 × 10⁻³ mol
The concentration of Fe²⁺ is: