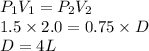Answer:
The answers are:
A = 1 L
B = 0.5 atm
C = 0.6 atm
D = 4 L
Step-by-step explanation:
Boyle's Law states that if the temperature and number of moles of gas are constant, then pressure (P) and volume (V) vary according to the relation:
PV = constant
To find A:
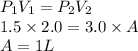
To find B:
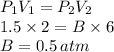
To find C:
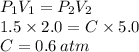
To find D: