Answer: The value of equilibrium constant for the reaction is

Step-by-step explanation:
The given chemical equation follows:
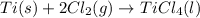
The expression of
 for the above reaction follows:
for the above reaction follows:
![K_(eq)=(1)/([Cl_2]^2)](https://img.qammunity.org/2020/formulas/chemistry/college/oya8090ucnasvkw5po5h6siyph9094c9ur.png)
Concentration of pure solids and liquids are taken as 1 in the equilibrium constant expression.
Thus, titanium and titanium tetrachloride does not appear in the expression.
We are given:
Equilibrium amount of titanium = 2.93 g
Equilibrium amount of titanium tetrachloride = 2.02 g
Equilibrium amount of chlorine gas = 1.67 g
To calculate the number of moles, we use the equation:

Given mass of chlorine gas = 1.67 g
Molar mass of chlorine gas = 71 g/mol
Putting values in above equation, we get:
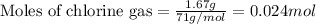
Volume of vessel = 7.0 L
Concentration of a substance is calculated by:
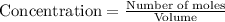
So, concentration of chlorine gas =
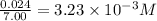
Putting the value in equilibrium constant expression, we get:
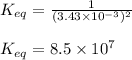
Hence, the value of equilibrium constant for the reaction is
